Lewis diagram for c2h6
Since C has 4 valence electronsand lewis diagram for c2h6 H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms.
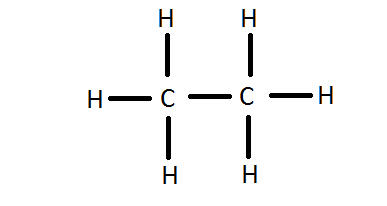
C 2 H 6 ethane has two carbon atoms and six hydrogen atoms. In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. Hence, carbon has four valence electrons and hydrogen has one valence electron. Learn how to find: Carbon valence electrons and Hydrogen valence electrons. We have a total of 14 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Lewis diagram for c2h6
In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now here the given molecule is C2H6 or ethane and it contains carbon atoms C and hydrogen atoms H. You can see the electronegativity values of carbon atom C and hydrogen atom H in the above periodic table. If we compare the electronegativity values of carbon C and hydrogen H then the hydrogen atom is less electronegative. But as per the rule we have to keep hydrogen outside.
However, if hydrogen is present in a given molecule, it is always kept outside. By doing so, you will get the following lewis structure of C2H6.
Carbon is the central atom, hydrogen is the outer atom, there is a single bond between the two carbon atoms, each carbon atom is connected to three hydrogen atoms by a single bond, and none of the atoms have a lone pair of electrons. The C2H6 Lewis structure is shown below:. Carbon and hydrogen are group 14 and group 1 elements in the periodic table. The central atom must satisfy the principle of less electronegativity. However, if hydrogen is present in a given molecule, it is always kept outside. So for the C2H6 or ethane molecule, even though the hydrogen atoms are less electronegative than the carbon atoms, we must leave the hydrogen on the outside. Thus, the carbon atom C is the central atom and the hydrogen atom H is the outer atom.
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V.
Lewis diagram for c2h6
In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3 -hybridized, meaning that both have four bonds arranged with tetrahedral geometry. The carbon-carbon bond, with a bond length of 1. All of these are sigma bonds. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. This means, in the case of ethane molecule, that the two methyl CH 3 groups can be pictured as two wheels on a hub, each one able to rotate freely with respect to the other. The sp 3 bonding picture is also used to described the bonding in amines, including ammonia, the simplest amine. Just like the carbon atom in methane, the central nitrogen in ammonia is sp 3 -hybridized.
Gibson zoot suit
Now in the C2H6 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-hydrogen atoms. Periodic table. This means that they have 8 electrons. Impact of this question views around the world. The outside atom left carbon also forms an octet, and all hydrogens form a duet. Therefore the central carbon atom is also stable. Hence, the octet rule and duet rule are satisfied. According to this theory, the shape and geometry of the molecule depend on the number of bonding electrons and lone pair of electrons. About author. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair. Also, in step 1 we have calculated the total number of valence electrons present in the C2H6 molecule.
Ethane is an organic compound with a chemical formula of C2H6.
Read more about our Editorial process. Carbon and hydrogen are group 14 and group 1 elements in the periodic table. Now here the given molecule is C2H6 or ethane and it contains carbon atoms C and hydrogen atoms H. During bond formation, the orbitals of atoms are hybridized to share electrons with another atom. By doing so, you will get the following lewis structure of C2H6. Visit our contact page. Related questions What are lewis dot structures used for? He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill. In addition, we must check that the central carbon atom C is stable, and we can see from the above steps that both carbon atoms are forming an octet. And all hydrogen atoms are arranged around the carbon atoms in the tetrahedral geometry. But as per the rule we have to keep hydrogen outside. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. In short, now you have to find the formal charge on carbon C atoms as well as hydrogen H atoms present in the C2H6 molecule. C 2 H 6 Lewis structure. In the above lewis dot structure of C2H6, you can also represent each bonding electron pair : as a single bond.

Sounds it is quite tempting
Also that we would do without your excellent idea