Lewis dot of h2s
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for pga leaderboard espn. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams, lewis dot of h2s. In all cases, the same lewis dot of h2s of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules.
There are 2 single bonds between the Sulfur atom S and each Hydrogen atom H. There are 2 lone pairs on the Sulfur atom S. In order to find the total valence electrons in H2S molecule , first of all you should know the valence electrons present in hydrogen atom as well as sulfur atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table.
Lewis dot of h2s
Hydrogen sulfide H2S consists of two hydrogen H atoms and one sulfur S atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom. Determining the Total Valence Electrons. To accurately represent the H2S Lewis structure, we need to calculate the total valence electrons. Sum the valence electrons of each atom:. Identify the Central Atom. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. Place the sulfur atom S at the center, surrounded by hydrogen atoms H. Sulfur forms single bonds with each hydrogen atom, utilizing two valence electrons for each bond. This results in a stable and balanced initial structure. Distributing Remaining Valence Electrons. Hydrogen atoms only require two electrons to achieve a full outer shell, while sulfur needs eight electrons. Begin by adding lone pairs dots around each hydrogen atom, fulfilling their duet rule. Next, distribute the remaining electrons around the sulfur atom to complete its octet.
The benefits, uses and side effects of L-Histidine. There are two single bonds in the molecule. Formal charges aid in determining the most plausible Lewis structure.
The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a The sulphur atom S and the two hydrogen atoms H are each connected by a single bond. The Lewis structure of H2S is shown below:. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. Therefore, there are 6 valence electrons in a sulphur atom and 1 valence electron in a hydrogen atom.
Hydrogen sulfide H 2 S is a gas with a foul smell, often described as being similar to rotten eggs. It is composed of two hydrogen atoms and one sulfur atom, and is important in various industrial processes and biochemical reactions. To draw the Lewis structure of hydrogen sulfide, follow these step-by-step instructions. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide, add the number of valence electrons in each atom. Hydrogen has one valence electron, while sulfur has six valence electrons. Therefore, H 2 S has a total of eight valence electrons. In hydrogen sulfide, sulfur is the central atom, since it is the atom with the highest valency. Hydrogen can only form one bond, while sulfur can form up to six bonds, so sulfur is capable of accommodating two hydrogen atoms.
Lewis dot of h2s
Transcript: All right, this is Dr. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that by 2. Plus Sulfur is in group 6 or 16 on the periodic table, so it has 6 valence electrons. Total of 8 valence electrons. Let's draw this thing.
Lista de dulces para aguinaldos
Therefore, there are 6 valence electrons in a sulphur atom and 1 valence electron in a hydrogen atom. Jay Rana. Hydrogen sulfide is important in the regulation of many cellular processes and is strongly implicated in oxygen sensing. Yes, H2S is a polar molecule due to the asymmetrical distribution of atoms and lone pairs, resulting in an overall dipole moment. This results in a stable and balanced initial structure. If we compare the electronegativity values of hydrogen H and sulfur S then the hydrogen atom is less electronegative. This indicates that the sulfur S and hydrogen H are chemically bonded with each other in a H2S molecule. You can see the number of bonding electrons and nonbonding electrons for each atom of H2S molecule in the image given below. H2S does not exhibit resonance structures since there are no multiple bond placements or delocalized electrons within the molecule. Adjust the arrangement of electrons as needed to minimize formal charges. In order to find the total valence electrons in H2S molecule , first of all you should know the valence electrons present in hydrogen atom as well as sulfur atom.
The hydrogen sulfide chemical formula is H2S. Drawing H2S Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct H2S Lewis Structure.
Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Table of Content. Step 4 Stability of the structure In order for the central sulphur S atom to be stable, we must check that it has an octet. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. This indicates that the sulfur S and hydrogen H are chemically bonded with each other in a H2S molecule. An octet rule governs Lewis structures. Leave a Comment Cancel Reply Your email address will not be published. Jay Rana. Valence electrons are the electrons that are present in the outermost orbit of any atom. Trending Questions. The stability of lewis structure can be checked by using a concept of formal charge. Sulfur forms single bonds with each hydrogen atom, utilizing two valence electrons for each bond. In the Lewis structure of H2S hydrogen sulfide , the sulfur S atom forms an octet with 8 electrons in its valence shell.

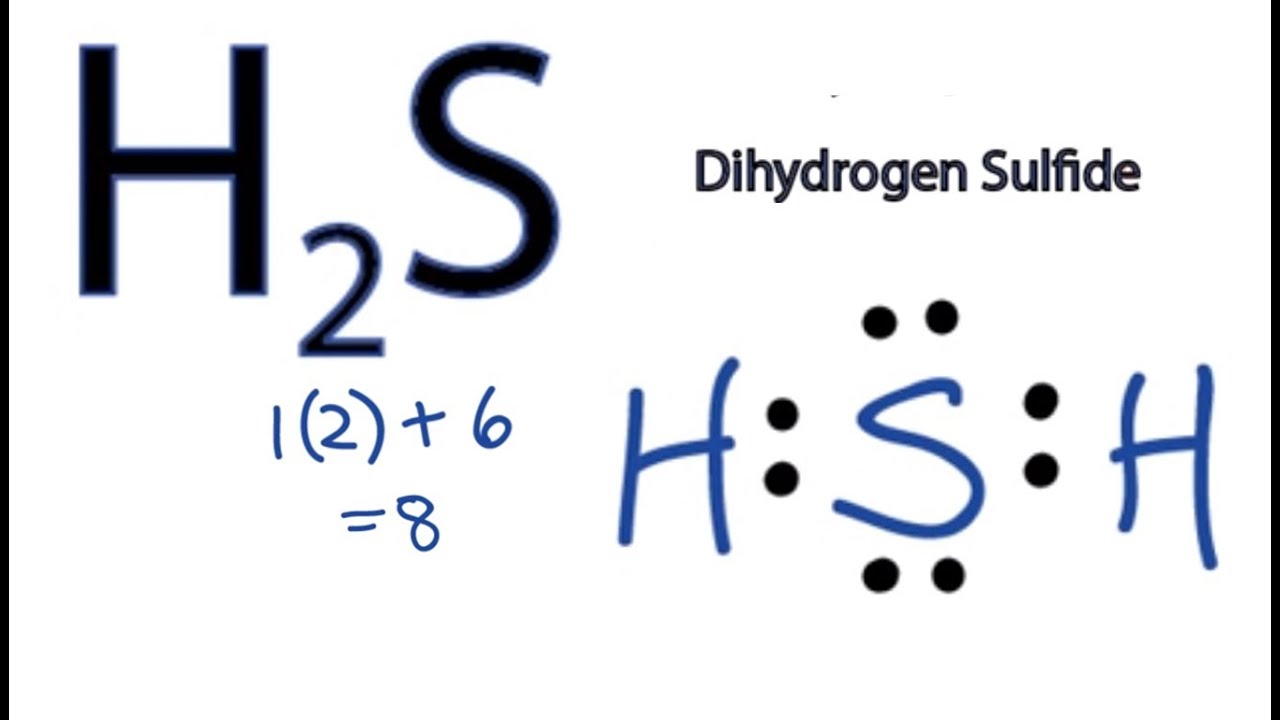
Can be.
It is a lie.
I confirm. All above told the truth. Let's discuss this question. Here or in PM.