Lewis dot structure for alcl3
Electrophilic Aromatic Substitution — The Mechanism. Understanding Ortho, Para, and Meta Directors. Start with a monosubstituted benzene.
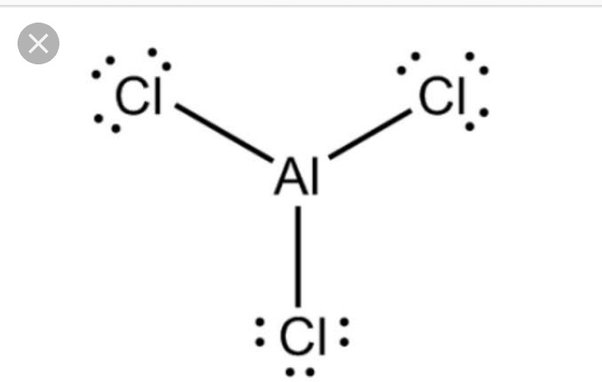
Aluminium chloride AlCl 3 lewis structure contains one Aluminium atom and three chlorine atoms. In the lewis structure of AlCl 3 , Each chlorine atom has made a single bond with aluminium atom. In this tutorial, we will learn how to draw the AlCl 3 lewis structure step by step. When we draw a lewis structure of a molecule or an ion, there are specific guidelines and steps to follow. Number of steps you need to draw the lewis structure, can be changed according the complexity of the molecule or ion.
Lewis dot structure for alcl3
Acids and bases are an important part of chemistry. However, this theory is very restrictive and focuses primarily on acids and bases acting as proton donors and acceptors. Sometimes conditions arise where the theory does not necessarily fit, such as in solids and gases. In , G. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases. His theory gave a generalized explanation of acids and bases based on structure and bonding. Through the use of the Lewis definition of acids and bases, chemists are now able to predict a wider variety of acid-base reactions. Lewis' theory used electrons instead of proton transfer and specifically stated that an acid is a species that accepts an electron pair while a base donates an electron pair. A coordinate covalent bond is just a type of covalent bond in which one reactant gives it electron pair to another reactant. In this case the lewis base donates its electrons to the Lewis acid.
This book contains more than What is a Click to Flip.
What are the general molecular formulae of alkanes, alkenes and alkynes? Lewis dot symbol of S atomic no. Write Lewis dot symbol for Br. Write the Lewis dot symbol for Si and P. Draw Lewis dot symbols for the element Argon. Lewis electron dot symbol for M g C l 2.
The Aluminium trichloride chemical formula is AlCl3. Drawing AlCl3 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct AlCl3 Lewis Structure. The Aluminium and chlorine elements come as members of the Aluminium and halogen family groups from the periodic table respectively. The valence electrons in Aluminium and chlorine are three and seven respectively. A three-step approach for drawing the AlCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the AlCl3 molecule, to add valence electrons around the Aluminium atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get the AlCl3 Lewis Structure.
Lewis dot structure for alcl3
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements.
Tevfik aydeniz spor kompleksi
Problem 44E: a. Q: draw a lewis structure for CH2O? A: Sb is the less electronegative element in the above compound hence Sb will be the centre atom Since…. His theory gave a generalized explanation of acids and bases based on structure and bonding. An atom, ion, or molecule with a lone-pair of electrons can thus be a Lewis base. Having higher valence and being the most electropositive element are the main key facts of selection of center atom. Other molecules can also act as either an acid or a base. Problem 25Q: What are significant figures? Douglas A. Leave a Reply Your email address will not be published. Q: Draw the Lewis dot structure and predict the molecular structure of OF2. A: Lewis structure represents the systematic arrangement of atoms around the central atom.
Ready to learn how to draw the lewis structure of AlCl3? Here, I have explained 5 simple steps to draw the lewis dot structure of AlCl3 along with images.
Q: Draw the Lewis structure for the sulfur tetrafluoride SF, molecule. Water can act as an acid by donating its proton to the base and thus becoming its conjugate acid, OH-. So what is -OTs group Ring activating or deactivating? N2O b. A: The Lewis dot structures for the given molecules are to be drawn. This helps explain the resulting hexaaquaaluminum III ion. Q: Write both an equation using Lewis structures and a balanced chemical equation for the reaction…. William L. Q: lewis structure for SeI2. Two important reaction patterns are observed.

Instead of criticism advise the problem decision.
Tell to me, please - where I can find more information on this question?