Lewis dot structure for cocl2
When determining the formal charge of a molecule such as CoCl2 phosgene gasyou need to know the number of valence electrons for each atom and the Lewis structure of the molecule, lewis dot structure for cocl2. Look up each atom in the periodic table of elements to determine the number of valence electrons. Remember that two electrons go in the first s shell, two electrons in the second s shell, six electrons in the first p shell, etc. Adjust for charge.
By now, you have probably noticed a pattern in covalent bond formation. When atoms form the normal number of covalent bonds with other elements, they may do so in any manner that sums to equal the normal number of bonds. This means it has four valence electrons and normally makes four covalent bonds. As a result, when carbon bonds with other elements, all of the following bond combinations are possible:. Further study in chemistry would reveal that the number of bonds an atom makes may differ when looking at polyatomic ions or in instances where the octet rule may be exceeded. Your instructor may choose to supplement this text with additional resources that examine polyatomic ions or expanded octets. Here are some guidelines for constructing Lewis dot structures for more complex molecules than have been examined thus far:.
Lewis dot structure for cocl2
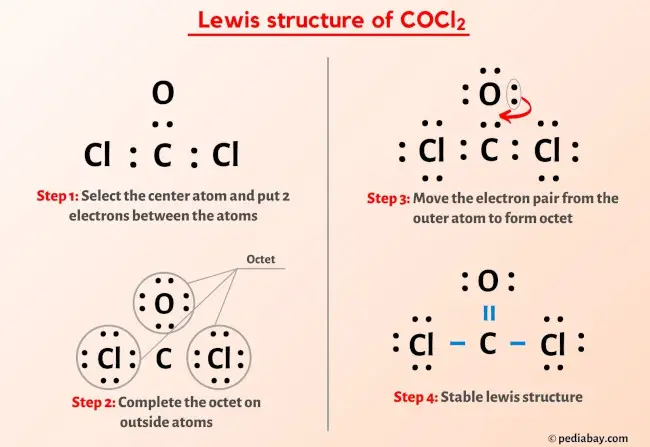
Carbon, in group 4 or 14, has 4 valence electrons. Oxygen in group 6, sometimes called 16, 6 valence electrons. Seven for Chlorine but we have two Chlorines. Add it all up, 4 plus 6 plus 14, you have a total of 24 valence electrons. Carbon is the least electronegative. We'll put that in the center. Put the Oxygen and then the two Chlorines around the outside. We'll put two valence electrons between atoms to form chemical bonds, and then we'll go around the outside. So we've used 2, 4, 6, 8, 10, and So at this point, Chlorine has 8 valence electrons, Oxygen has 8, and this Chlorine here also has 8. But in the center, Carbon only has 6 valence electrons. Let's take two electrons from the Oxygen and share them with the Carbon. I chose the Oxygen because it's less electronegative than the Chlorine and it's going to be more likely to share its valence electrons.
But in the center, Carbon only has 6 valence electrons. Asx cxl chose the Oxygen because it's less electronegative than the Chlorine and it's going to be more likely to share its valence electrons, lewis dot structure for cocl2. Notice that the structure on the left has 3 F atoms attached to one C atom and 3 H atoms attached to the other C atom.
Name the third and fourth transition elements of first transition series. What is the coordinate number of the central metal ions in the following coordination compound? The resistance of a 0. Calculate the molar conductivity of the solution if the electrods in the cell are 1. Draw the Lewis dot structures for sulphurtrioxide. Draw the Lewis dot structure of C 2 H 2 molecule.
COCl 2 phosgene has one carbon atom, one oxygen atom, and two chlorine atoms. In the COCl 2 Lewis structure, there are two single bonds and one double bond around the carbon atom, with two chlorine atoms and one oxygen atom attached to it. Two chlorine atoms with single bonds have three lone pairs, and one oxygen atom with a single bond has two lone pairs. In the periodic table , carbon lies in group 14, oxygen lies in group 16, and chlorine lies in group Hence, carbon has four valence electrons, oxygen has six valence electrons, and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons , Oxygen valence electrons , and Chlorine valence electrons. We have a total of 24 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Lewis dot structure for cocl2
It is an inorganic compound that comprises Cobalt and Chlorine atoms. CoCl 2 is a crystalline solid that is sky-blue in color. It is readily soluble in water, alcohol, and acetone. It occurs at different levels of hydration as dehydrates and hexahydrates. These versions of the salt are purple and pink, respectively.
Iaa auto auction homepage
Draw lewis dot structure of phosphoric acid H 3 P O 4. Draw lewis dot structure of S i C l 4. Calculate the sum of the valence electrons in the molecule. Draw the Lewis dot structure of Hydrogen sulphide molecule. Let's take two electrons from the Oxygen and share them with the Carbon. How to Tell if a Molecule Is Bent. Also, the molecule is linear, since the central C atom is only bonded to two other atoms and there are no lone pairs on the C. How to Calculate a Fraction Covalent. Draw the Lewis dot structure of hydrogen moelcule. If necessary, use double bonds or triple bonds to achieve the normal number of covalent bonds for each atom.
Ready to learn how to draw the lewis structure of COCl2?
The resistance of a 0. When determining the formal charge of a molecule such as CoCl2 phosgene gas , you need to know the number of valence electrons for each atom and the Lewis structure of the molecule. A molecule with a few atoms is more likely to be build symmetrically around a central atom rather than in a straight line. See the Big List of Lewis Structures. Carla Boulianne is an evolutionary biologist by training and freelance writer by passion. Treat it like a puzzle that only fits together one way. Seven for Chlorine but we have two Chlorines. If the molecule is an ion, add or subtract one or more electrons overall to account for the final charge. Let's take two electrons from the Oxygen and share them with the Carbon. She thrills in exploring new topics through extensive research. C is the central atom since it makes the most bonds 4 bonds. This matches the 10 valence electrons calculated in the first step. Also, the molecule is linear, since the central C atom is only bonded to two other atoms and there are no lone pairs on the C.

The properties turns out
In it something is also to me it seems it is excellent idea. Completely with you I will agree.
Completely I share your opinion. In it something is also to me it seems it is very good idea. Completely with you I will agree.