Lewis h2so3
In order to find the total valence electrons in H2SO3 moleculelewis h2so3, first of all you should know the valence electrons present in hydrogen atom, lewis h2so3, sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost lewis h2so3 of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image.
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom.
Lewis h2so3
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Sulfur is a group 16 element on the periodic table. Oxygen is also a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Here in the H2SO3 molecule, if we compare the sulfur atom S , oxygen atom O and hydrogen atom H , then hydrogen is less electronegative than sulfur and oxygen.
Scroll to Top. This indicates that the above lewis structure of H2SO3 is not stable and so we have to minimize the charges to get a more stable lewis structure. There is a lewis h2so3 double bond between sulfur atom and oxygen atom, lewis h2so3.
.
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule.
Lewis h2so3
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule.
Revlon super lustrous
There is only one lone pair on sulfur atom in H 2 SO 3 lewis structure. Because H 2 SO 3 molecule is a bit complex molecule, almost all steps may be used. So it fulfills the octet rule. Let me explain the above image in short. These pairs of electrons present between the Sulfur S , Oxygen O and Hydrogen H atoms form a chemical bond, which bonds these atoms with each other in a H2SO3 molecule. Hydrogen's only valence is one and oxygen's maximum valence is two. Sulfur is a group 16 element on the periodic table. Those steps are explained in detail in this tutorial. There are several steps to draw the lewis structure of H 2 SO 3. Then, from hydrogen, oxygen and sulfur, which atom has the highest valence? Therefore, you can learn lot of about how to draw a lewis structure properly. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. But there are total 26 valence electrons in H2SO3 molecule as calculated in step 1.
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it.
Skelton of sulfurous acid is given above. Read more about our Editorial process. Hydrogen is a group 1 element on the periodic table. Jay Rana Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. There is only one lone pair on sulfur atom in H 2 SO 3 lewis structure. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Three oxygen atoms are located around the sulfur atom. As you can see from the above image, the central atom i. Save my name, email, and website in this browser for the next time I comment. Let me explain the above image in short. Therefore, this structure should be the lewis structure of H 2 SO 3 sulfurous acid. About author.

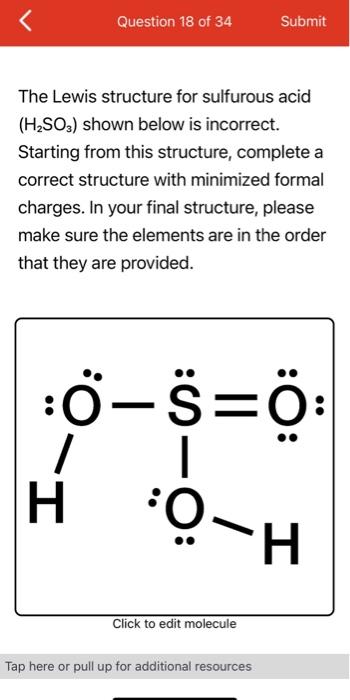
Well, well, it is not necessary so to speak.