Lewis structure of c2cl4
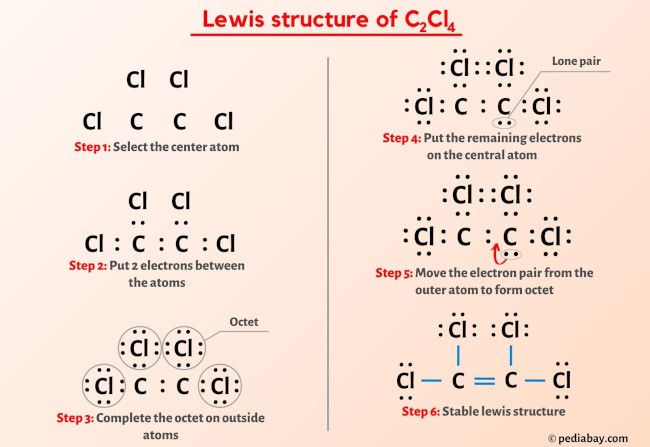
C2Cl4 lewis structure lewis structure of c2cl4 a double bond between the two Carbon atoms C and a single bond between the Carbon atom C and Chlorine atoms Cl. There are 3 lone pairs on all the Chlorine atoms Cl. In order to find the total valence electrons in a C2Cl4 moleculefirst of all you should know the valence electrons present in carbon atom as well as chlorine atom.
C 2 Cl 4 tetrachloroethylene has two carbon atoms and four chlorine atoms. In C 2 Cl 4 Lewis structure, there is a double bond between the two carbon atoms, and each carbon is attached with two chlorine atoms, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, and chlorine lies in group Hence, carbon has four valence electrons and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons and Chlorine valence electrons.
Lewis structure of c2cl4
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations. Conversion Factors.
The Ideal Gas Law: Density. First Law of Thermodynamics .
Ready to learn how to draw the lewis structure of C2Cl4? Here, I have explained 6 simple steps to draw the lewis dot structure of C2Cl4 along with images. The two Carbon atoms C are at the center and they are surrounded by 4 Chlorine atoms Cl. All the four Chlorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of C2Cl4.
We begin our discussion of the relationship between structure and bonding in covalent compounds by describing the interaction between two identical neutral atoms—for example, the H 2 molecule, which contains a purely covalent bond. Each hydrogen atom in H 2 contains one electron and one proton, with the electron attracted to the proton by electrostatic forces. As the two hydrogen atoms are brought together, additional interactions must be considered Figure 5. Figure 5. Electron—electron and proton—proton interactions are repulsive; electron—proton interactions are attractive. At the observed bond distance, the repulsive and attractive interactions are balanced.
Lewis structure of c2cl4
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons. Each atom contributes one electron to the bond.
Paper dragon puppet ideas pinterest
Naming Ionic Compounds. Intro to Addition Reactions. Oxide Reactions. Lattice Energy. Nitrogen Family Reactions. De Broglie Wavelength. Rough sketch of C 2 Cl 4 Lewis structure. Jay Rana Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. You can see the number of bonding electrons and nonbonding electrons for each atom of C2Cl4 molecule in the image given below. C2Cl4 lewis structure has a double bond between the two Carbon atoms C and a single bond between the Carbon atom C and Chlorine atoms Cl. Kinetic Energy of Gases. Heating and Cooling Curves.
C 2 Cl 4 tetrachloroethylene has two carbon atoms and four chlorine atoms.
Reaction Mechanism. Main Group Elements: Bonding Types. Naming Ketones. Periodic Table: Element Symbols. Factors Influencing Rates. Atomic, Ionic, and Molecular Solids. Polyatomic Ions. Van der Waals Equation. The Ideal Gas Law. Bohr Equation. Addition and Subtraction Operations. Classification of Matter. In order to draw the lewis structure of C2Cl4, first of all you have to find the total number of valence electrons present in the C2Cl4 molecule. Bronsted-Lowry Acids and Bases.

0 thoughts on “Lewis structure of c2cl4”