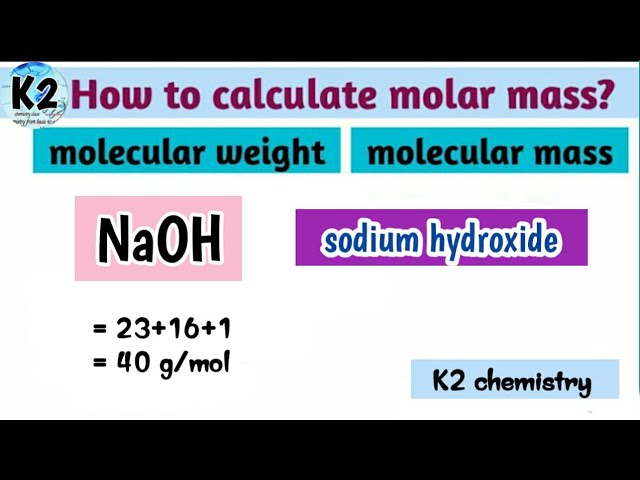
Molar mass of sodium hydroxide
Molar mass of NaOH Sodium hydroxide is Then, lookup atomic weights for each element in periodic table : Na: Weights of atoms and isotopes are from NIST article.
Sodium hydroxide , also known as lye and caustic soda , [1] [2] is an inorganic compound with the formula NaOH. Sodium hydroxide is a highly corrosive base and alkali that decomposes lipids and proteins at ambient temperatures and may cause severe chemical burns. It is highly soluble in water , and readily absorbs moisture and carbon dioxide from the air. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper , textiles , drinking water , soaps and detergents , and as a drain cleaner.
Molar mass of sodium hydroxide
.
Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. External SDS. The sodium hydroxide-based detergents include surfactants, rust inhibitors and defoamers.
.
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles. You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula. To understand the topic as a whole, you will want to learn the mole definition, read a paragraph about the molarity units, as well as read a comparison of two misleading concepts: molarity formula vs molality formula. What is more, we prepared for you some interesting examples of molar solutions and a short step-by-step tutorial on how to calculate the molarity of a concentrated solution.
Molar mass of sodium hydroxide
Molar mass of NaOH Sodium hydroxide is Then, lookup atomic weights for each element in periodic table : Na: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element.
Titanic toys
Interactive image. Nd OH 3. Sodium hydroxide is used to detect the presence of flavonoids. The "sodium hydroxide" of commerce is often the monohydrate density 1. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. For example, when a solution of NaOH and water with mole ratio Periodic table. Qily is the ashes of certain plants, e. Find atomic masses: look up the atomic masses of each element present in the compound. Sodium hydroxide is used in many scenarios where it is desirable to increase the alkalinity of a mixture, or to neutralize acids. Sodium hydroxide is a dangerous chemical due to its ability to hydrolyze protein.
You can go from moles of sodium hydroxide, "NaOH" , to grams of sodium hydroxide by using a conversion factor called molar mass. The molar mass tells you the mass of exactly one mole of a given substance.
Sodium hydroxide is used in some cement mix plasticisers. Journal of the Chemical Society, Transactions. Yb OH 3. Archived from the original on Carcass Disposal: A Comprehensive Review. Formation of sodium tetrahydroxoaluminate III or hydrated sodium aluminate is given by: [38]. A treatise on chemistry applied to the manufacture of soap and candles. Archived from the original on May 28, Sodium hydroxide is often stored in bottles for small-scale laboratory use, within intermediate bulk containers medium volume containers for cargo handling and transport, or within large stationary storage tanks with volumes up to , gallons for manufacturing or waste water plants with extensive NaOH use. Chemical compound with formula NaOH. Weinheim: Wiley-VCH. Sodium hydroxide is a dangerous chemical due to its ability to hydrolyze protein. Moreover, dissolution of sodium hydroxide is highly exothermic , and the resulting heat may cause heat burns or ignite flammables. Archived from the original on July 1,

It is a pity, that now I can not express - it is very occupied. I will be released - I will necessarily express the opinion.
I think, what is it � error. I can prove.