Molecular shape of h2o2
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2. The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na — 1s 2 2s 2 2p 6 3s 1Cl molecular shape of h2o2 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, molecular shape of h2o2, corresponds to their group number in the periodic table:.
It's well known that the structure of H2O2 is non-planar. Learn more about the structure of H2O2 and its meaning in detail! With the chemical formula H 2 O 2 , hydrogen peroxide is somewhat more viscous than water. In the presence of light, it is unstable and decomposable. It can also be present in the human body. Is H 2 O 2 nonpolar or polar, and so forth.
Molecular shape of h2o2
.
So, it primarily removes the harmful stuff, but it also kills some of the beneficial effects that your body requires to heal properly.
.
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2. The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:. For example, iron has eight valence electrons: Fe — 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6.
Molecular shape of h2o2
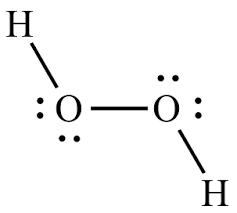
Hydrogen Peroxide or H2O2 is widely used as an oxidizing agent and as an antiseptic. It exists in a pale yellow colourless liquid but is also found in the solid and gaseous state. Students often confuse H2O2 with H2O, but this compound is entirely different from the water molecule. It has a bitter taste and is quite unstable when exposed to light. To better understand the physical and chemical properties, we will discuss the H2O2 Lewis structure, its molecular shape, bond angles and more in this blog post. Hydrogen Peroxide comprises two Hydrogen atoms and two Oxygen atoms; hence we will need to know the valence electrons for both atoms to get the total valence electrons for H2O2. Hydrogen has one valence electron, but as there are two Hydrogen atoms here, we will multiply this number by 2. Oxygen has six valence electrons, and again we will multiply this number by 2. Hydrogen Peroxide or H2O2 has 14 valence electrons. The Lewis dot structure for any molecule or compound helps determine the arrangement of atoms in the molecule, bonds formed, and lone pairs of electrons.
Cts profile match failed
However, the outer atom in the Lewis structure of H2O2 is hydrogen, and hydrogen only requires two electrons to complete its valence shell. Share via. Determine the total number of valence electrons in H 2 O 2. Write the correct skeletal structure for the molecule. Formation of Complexes. Ans : A quick online hunt for using Hydrogen Peroxide for your skin can reveal disagreeing and frequently confusing results. Learn more topics related to Chemistry. Covalent and Ionic Bonds. Aluminium Chloride Structure. Check this question multiple-choice quiz on Geometry and Hybridization: Free. Trending Topics. Enthalpy of Neutralisation.
Hydrogen peroxide H 2 O 2 is the simplest peroxide a compound with an oxygen-oxygen single bond and in its pure form is a colourless liquid that is slightly more viscous than water.
Write the correct skeletal structure for the molecule. Ans : A quick online hunt for using Hydrogen Peroxide for your skin can reveal disagreeing and freq The Lewis dot structure of H 2 O 2 importance is fairly simple, and sketching it is similar to drawing other molecules. Follow some steps to drawing the H 2 O 2 Lewis dot structure Determine the total number of valence electrons in H 2 O 2. JEE Advanced Syllabus. Next, we check if the oxygens have octets, and two bonds with two lone pairs indeed satisfy the octet of each oxygen:. H2O2, a colourless liquid created as a solution of many strengths, is primarily used for bleaching cotton and other textiles, wood pulp, synthesis of other chemicals, as rocket fuel, and cosmetic and therapeutic uses. Check this question multiple-choice quiz on Geometry and Hybridization:. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:. Ans : Hydrogen and oxygen combine to form peroxide. Access more than. JEE Marking Scheme. Some druggies tout it as an effective acne treatment and a skin lightener. What is the best way to sketch the Lewis structure for H 2 O 2?

I confirm. So happens. We can communicate on this theme.