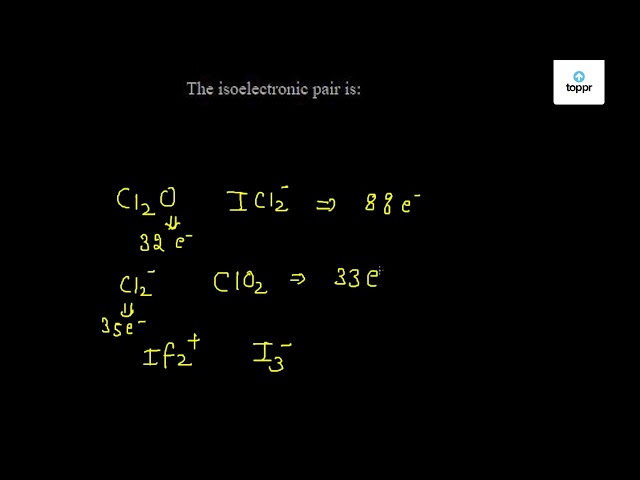
The isoelectronic pair is
Isoelectronic means "same electronic structure".
Isoelectronic refers to two ions or molecules having the same electronic structure and the same number of valence electrons. Last updated on Dec 20, Candidates must go through the NDA1 previous year's papers. Attempting the NDA1 mock tests is also essential. Get Started.
The isoelectronic pair is
To state whether the given species are isoelectronic or not we have to calculate the number of electrons in both. Key Points. Last updated on Jan 2, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams. Defence Exams.
Was this answer helpful? Delhi Home Guard. The cooking gas is mainly a mixture of the following two gases:.
Courses for Kids. Free study material. Offline Centres. Talk to our experts Last updated date: 22nd Feb Study Material. Important Questions.
Species such as atoms, molecules, or ions having the same number of electrons are called isoelectronic species. It is quite easy to identify isoelectronic species either by counting the total number of electrons or by writing electronic configuration. The electronic configuration of atoms or ions can be easily written but that of molecules is slightly difficult. Thus, I prefer to identify such species by counting electrons. I have tried to present simple way for the calculation of electrons in either atom, ions or molecules below. As stated above, these species have the same number of electrons, right? But these have different atomic number different number of proton. S 2- ion has 16 protons in its nucleus and 18 electrons in shell whereas Cl — ion has 17 protons and 18 electrons.
The isoelectronic pair is
Atomism, because it was dismissed by Aristotle, enjoyed a long sleep in scientific discourse until it was reconsidered by Galileo, Decartes, and Gassendi in the s. Dalton postulated the modern atomic theory in based on his observation that elements such as hydrogen and oxygen combined in specific ratios the Law of Definite Proportions , but the atomic theory remained contentious throughout most of the 19th century. Thompson, Rutherford, Bohr, and others around the turn of the 20th century established that matter was indeed composed of atoms that contained heavy nuclei and light electrons, and that atoms could exist in excited states that could be interpreted as excitations of their electrons to different energy levels. However the atomic theory did not provide a ready explanation for the bonded states of atoms in molecules. In , still more than a decade before modern quantum theory would adequately describe the shapes of atomic orbitals, Lewis proposed the octet theory based on the empirically observed rules of valence, i. In Lewis' model, the valence electrons of an atom were situated at the corners of a cube, and the cubes could share edges or faces to complete their octets. Lewis developed a shorthand notation for these structures based on dots that represented the valence electrons, as illustrated in Fig. A pair of electrons shared between atoms constitutes a chemical bond, and can also be represented as a line joining the atoms.
Desi twitter
Navy AA. The isoelectronic pair of ions is -. The total number of electrons in Fe is Punjab Superior Judicial Service. Chhattisgarh Junior Engineer. IB Junior Intelligence Officer. The isoelectronic pair of 32 electrons is. MP Vyapam Group 2. Rajasthan Pre D. Which method can be employed to produce a high degree of homogeneity in the creation of ZnFe2O4 spinel? Previous Year Question Paper.
An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion cation or when additional electrons attach themselves to neutral atoms to form a negative one anion. The designations cation or anion come from the early experiments with electricity which found that positively charged particles were attracted to the negative pole of a battery, the cathode, while negatively charged ones were attracted to the positive pole, the anode. Ionic compounds consist of regular repeating arrays of alternating positively charged cations and negatively charges anions.
Chandigarh Police ASI. Bihar Sakshamta Pariksha. CISF Driver. ACC Exam. Bihar Elementary Teacher. The role of haemoglobin is to. HPCL Technician. Was this answer helpful? Gujarat High Court Assistant. Maharashtra Agriculture Assistant. Uttarakhand Patwari. Which gas is used for the preparation of soda water? AAI Junior Assistant.

You are not right. I am assured. Let's discuss.
I am sorry, that I interfere, I too would like to express the opinion.
I am sorry, that I interrupt you, I too would like to express the opinion.